Trans fat

| Types of fats in food |
|---|
| Components |
| Manufactured fats |
Trans fat is a type of unsaturated fat that occurs in foods.[1][2] Small amounts of trans fats occur naturally, but large amounts are found in some processed foods made with partially hydrogenated oils.[1][2] Because consumption of trans fats is associated with increased risk for cardiovascular diseases, artificial trans fats are highly regulated or banned in many countries.[3][4][5][6][7][8] However, they are still widely consumed in developing nations where they are associated with increased risk of diabetes, cardiovascular diseases, and death.[9]
In 2015, the US Food and Drug Administration (FDA) stated that artificial trans fats from partially hydrogenated oils were not generally recognized as safe (GRAS), and the use of such oils and trans fats should be limited or eliminated from manufactured foods.[2] Numerous countries, including the European Union, Canada, and Australia/New Zealand, followed with restrictions or bans on the use of partially hydrogenated oils and trans fats in food manufacturing.[1][7][10] The World Health Organization (WHO) had set a goal to make the world free from industrially produced trans fat by the end of 2023.[11] The goal was not met, and the WHO announced another goal in 2024 "for accelerated action till 2025 to complete this effort".[8]
Trans fatty acids (also called trans-unsaturated fatty acids) are derived from trans fats, which are triglycerides (esters of glycerin). Trans fats are converted to trans fatty acids in the digestive tract prior to absorption.
Occurrence
[edit]Trans fats occur naturally in the fats of products made from ruminant animals, such as cheese or butter, and some are used in manufactured foods, such as cooking oils or margarine.
Naturally-occurring trans fats
[edit]
Trans fats occur in meat and dairy products from ruminants. For example, butter contains about 3% trans fat by weight.[12] These naturally occurring trans fats include conjugated linoleic acid (CLA) and vaccenic acid. They arise from the action of bacteria in the rumen. Polyunsaturated fats are toxic to the rumen-based bacteria, which detoxify the fats by changing some cis-double bonds to trans-double bonds. In contrast to industrially produced trans fats, this bacterial process produces only a few specific isomers. As industrial sources of trans fats are eliminated, increased attention focuses on ruminant derived trans fats.[13]
Small amounts of trans fats occur in meat and milk fat.[14]
Hydrogenation
[edit]Trans fat can be an unintentional byproduct of the industrial processing of oils. Unlike naturally derived trans fats, the trans fats that result from hydrogenation consist of many isomers. In food production, liquid cis-unsaturated fats such as vegetable oils are hydrogenated to produce more saturated fats, which have desirable properties:
- The shelf life of fats correlates with the degree of saturation: polyunsaturated fats are prone to autoxidation whereas saturated fats, being virtually inert in air, have very long shelf lives.
- Saturated fats tend to be more solid at room temperature. This property is important for margarine, one of the original uses for fat hydrogenation.
However, an isomerization side reaction during fat hydrogenation can convert remaining unsaturated fats to the thermodynamically-favored trans isomer.

A number of old and new ingredients are available to replace partially-hydrogenated oil containing significant levels of trans fat. These include partially-hydrogenated oil made with improved processes, plant oils rich in monounsaturated fats and saturated fats, and a mix of fats combined with interesterification.[15] The technology has improved such that a 2021 review indicates that trans fat from hydrogenated fats is no longer a problem in modern countries.[13]
Thermal isomerization
[edit]When heated (cooked), some unsaturated fats change from their normal geometry to trans. The rate of isomerization is accelerated by free radicals.[16][17][18]
History
[edit]There were suggestions in the scientific literature as early as 1956 that trans fats could cause an increase in coronary artery disease.[19] Studies in the early 1990s brought renewed scrutiny and confirmation of the negative health impact of trans fats. In 1994, it was estimated that trans fats caused at least 20,000 deaths annually in the U.S. from heart disease.[20] In the 1990s, activists such as CSPI that had promoted trans fat safety began arguing that trans fats should be disclosed on product labels and menus.[22] Several lawsuits were launched against high-visibility restaurants and food manufacturers with the objective of supporting a broader phase-out of trans fats.[23][24]
Mandatory food labeling was introduced in several countries[25] and Denmark was first to mandate limits on industrially-produced trans fats in 2004.[26] In January 2007, faced with the prospect of an outright ban on the sale of their product, Crisco was reformulated to meet the U.S. Food and Drug Administration (FDA) definition of "zero grams trans fats per serving" (that is less than one gram per tablespoon, or up to 7% by weight; or less than 0.5 grams per serving size)[27][28][29][30] by boosting the saturation and then diluting the resulting solid fat with unsaturated vegetable oils. Noting that elimination of industrially produced trans fat is feasible and achievable, the World Health Organization (WHO) has set a goal to make the world free from industrially produced trans fat by the end of 2023. By the end of 2021, the WHO announced that 40 countries had implemented industrial trans fat elimination policies that "are protecting 1.4 billion people from this deadly food compound" but that 10 of the 15 countries suffering the highest health impacts from trans fats had not yet adopted a policy.[11]
Structure
[edit]A fatty acid is characterized as either saturated or unsaturated based on the respective absence or presence of C=C double bonds in its backbone. If the molecule contains no double C=C bonds, it is said to be saturated; otherwise, it is unsaturated to some degree.[31][32]
The C=C double bond is rotationally rigid. If the hydrogen bonded to each of the carbons in this double bond are on the same side, this is called cis, and leads to a bent molecular chain. If the two hydrogens are on opposite sides, this is called trans, and leads to a straight chain.
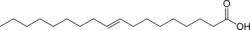
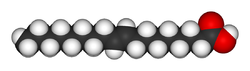
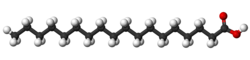
| Trans unsaturated (Elaidic acid) | Cis unsaturated (Oleic acid) | Saturated (Stearic acid) |
|---|---|---|
| Elaidic acid is the main trans unsaturated fatty acid often found in partially hydrogenated vegetable oils.[33] | Oleic acid is a cis unsaturated fatty acid making up 55–80% of olive oil.[34] | Stearic acid is a saturated fatty acid found in animal fats and is the intended product in full hydrogenation. Stearic acid is neither unsaturated nor trans because it has no carbon-carbon double bonds. |
 |
 |
|
 |
||
| These fatty acids are geometric isomers (structurally identical except for the arrangement of the double bond). | This fatty acid contains no carbon-carbon double bonds and is not isomeric with the prior two. | |
Because trans fats are more linear, they crystallize more easily, allowing them to be solid (rather than liquid) at room temperatures. This has several processing and storage advantages.
In nature, unsaturated fatty acids generally have cis configurations as opposed to trans configurations.[35] Saturated fatty acids (those without any carbon-carbon double bonds) are abundant (see tallow), but they also can be generated from unsaturated fats by the process of fat hydrogenation. In the course of hydrogenation, some cis double bonds convert into trans double bonds. Chemists call this conversion an isomerization reaction.[17][18][36]
The same molecule, containing the same number of atoms, with a double bond in the same location, can be either a trans or a cis fatty acid depending on the configuration of the double bond. For example, oleic acid and elaidic acid are both unsaturated fatty acids with the chemical formula C9H17C9H17O2.[37] They both have a double bond located midway along the carbon chain. It is the configuration of this bond that sets them apart. The configuration has implications for the physical-chemical properties of the molecule. The trans configuration is straighter, while the cis configuration is noticeably kinked as can be seen from the three-dimensional representation shown above. Cis- and trans fatty acids (and their derivatives) have distinct chemical (and metabolic) properties, For example, the melting point of elaidic acid is 45 °C higher than that of oleic acid.[37] This notably means that it is a solid at human body temperatures.
Hydrogenation as a source of trans fats
[edit]{{main|Fat hydrogenation]] The hydrogenation process was widely adopted by the food industry in the early 1900s; first for the production of margarine, a replacement for butter and shortening,[38] and eventually for various other fats used in snack food, packaged baked goods, and deep fried products.[18]
Full hydrogenation of a fat or oil produces a fully saturated fat. For food purposes, hydrogenation generally is not allowed to go to completion. The main target is a specific melting point and hardness, and this fine-tuning requires that some unsaturation remain. This partial hydrogenation turns some of the cis double bonds into trans bonds by an isomerization reaction.[18][39] This side reaction accounts for most of the trans fatty acids consumed today, by far.[40][41]
The standard 140 kPa (20 psi) process of hydrogenation produces a product of about 40% trans fatty acid by weight, compared to about 17% using higher pressures of hydrogen. Blended with unhydrogenated liquid soybean oil, the high-pressure-processed oil produced margarine containing 5 to 6% trans fat. Based on current U.S. labeling requirements (see below), the manufacturer could claim the product was free of trans fat.[42] The level of trans fat may also be altered by modification of the temperature and the length of time during hydrogenation.
The trans fat levels can be quantified using various forms of chromatography.[16]
Presence in food
[edit]| Food type | Trans fat content |
|---|---|
| shortenings | 10–33 |
| margarine, stick | 6.2–16.8[44] |
| butter | 2–7 |
| whole milk | 0.07–0.1 |
| breads/cake products | 0.1–10 |
| cookies and crackers | 1–8 |
| tortilla chips | 5.8[44] |
| cake frostings, sweets | 0.1–7 |
| animal fat | 0–5[45] |
| ground beef | 1 |
Animal fats
[edit]trans fatty acids (TFAs) occur in small amounts in meat and milk of ruminants (such as cattle and sheep),[46][47] typically 2–5% of total fat.[48] Natural TFAs, which include conjugated linoleic acid (CLA) and vaccenic acid, originate in the rumen of these animals. CLA has two double bonds, one in the cis configuration and one in trans, which makes it simultaneously a cis- and a trans-fatty acid.[49] A type of trans fat occurs naturally in the milk and body fat of ruminants (such as cattle and sheep) at a level of 2–5% of total fat.[45] Natural trans fats, which include conjugated linoleic acid (CLA) and vaccenic acid, originate in the rumen of these animals. CLA has two double bonds, one in the cis configuration and one in trans, which makes it simultaneously a cis- and a trans-fatty acid.
The US National Dairy Council has asserted that the trans fats present in foods of animal origin are of a different type than those in partially hydrogenated oils, and do not appear to exhibit the same negative effects.[50] A scientific review agrees with the conclusion (stating that "the sum of the current evidence suggests that the Public health implications of consuming trans fats from ruminant products are relatively limited") but cautions that this may be due to the low consumption of trans fats from animal sources compared to artificial ones.[44]
Despite this concern, the NAS dietary recommendations have not included eliminating trans fat from the diet. This is because trans fat is naturally present in many animal foods in trace quantities, and thus its removal from ordinary diets might introduce undesirable side effects and nutritional imbalances if proper nutritional planning is not undertaken. The NAS has, thus, "recommended that trans fatty acid consumption be as low as possible while consuming a nutritionally adequate diet".[51] Like the NAS, the World Health Organization has tried to balance public health goals with a practical level of trans fat consumption, recommending in 2003 that trans fats be limited to less than 1% of overall energy intake.[45]
A meta-analysis showed that all trans fats, regardless of natural or artificial origin equally raise LDL and lower HDL levels.[6] Other studies though have shown different results when it comes to animal based trans fats like conjugated linoleic acid (CLA). Although CLA is known for its anticancer properties, researchers have also found that the cis-9, trans-11 form of CLA can reduce the risk for cardiovascular disease and help fight inflammation.[52][53]
Natural "trans fats" in dairy products
[edit]Some trans fatty acids occur in natural fats and traditionally processed foods. Vaccenic acid occurs in breast milk, and some isomers of conjugated linoleic acid (CLA) are found in meat and dairy products from ruminants. Butter, for example, contains about 3% trans fat.[54]
The U.S. National Dairy Council has asserted that the trans fats present in animal foods are of a different type than those in partially hydrogenated oils, and do not appear to exhibit the same negative effects.[55] A review agrees with the conclusion (stating that "the sum of the current evidence suggests that the Public health implications of consuming trans fats from ruminant products are relatively limited") but cautions that this may be due to the low consumption of trans fats from animal sources compared to artificial ones.[44]
In 2008 a meta-analysis found that all trans fats, regardless of natural or artificial origin equally raise LDL and lower HDL levels.[6] Other studies though have shown different results when it comes to animal-based trans fats like conjugated linoleic acid (CLA). Although CLA is known for its anticancer properties, researchers have also found that the cis-9, trans-11 form of CLA can reduce the risk for cardiovascular disease and help fight inflammation.[52][56]
Processed foods
[edit]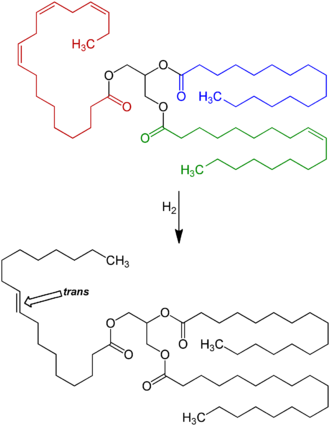
Partially hydrogenated vegetable oils were an increasingly significant part of the human diet for about 100 years, especially after 1950 as processed food rose in popularity.[41]
Animal-based fats were once the only trans fats consumed, but by far the largest amount of trans fat consumed today is created by the processed food industry as a side effect of partially hydrogenating unsaturated plant fats (generally vegetable oils). These partially hydrogenated fats have displaced natural solid fats and liquid oils in many areas, the most notable ones being in the fast food, snack food, fried food, and baked goods industries.[57]
Up to 45% of the total fat in those foods containing human-made trans fats formed by partially hydrogenating plant fats may be trans fat.[45][48] An analysis of some industrialized foods in 2006 found up to 30% "trans fats" in artificial shortening, 10% in breads and cake products, 8% in cookies and crackers, 4% in salty snacks, 7% in cake frostings and sweets, and 26% in margarine and other processed spreads.[58] Another 2010 analysis however found only 0.2% of trans fats in margarine and other processed spreads.[59]
Foods containing higher amounts of trans fat is associated with fast food restaurants.[41] They are consumed in greater quantities by people who lack access to a diet consisting of fewer partially-hydrogenated fats, or who often consume fast food. A diet high in trans fats can contribute to obesity, high blood pressure, and higher risk for heart disease. Trans fat is also implicated in Type 2 diabetes.[60]
Shortenings
[edit]Shortenings, because they are widely used, are of particular concern. Baking shortenings, unless reformulated, contain around 30% trans fats compared to their total fats. High-fat dairy products such as butter contain about 4%. Margarines not reformulated to reduce trans fats may contain up to 15% trans fat by weight,[61] but some reformulated ones are less than 1% trans fat. Shortenings for deep-frying in restaurants can be used for longer than most conventional oils before becoming rancid. In the early 21st century, non-hydrogenated vegetable oils that have lifespans exceeding that of the frying shortenings became available.[62] In fast-food chains, trans fat levels in fast food can vary with location. For example, an analysis of samples of McDonald's French fries collected in 2004 and 2005 found that fries served in New York City contained twice as much trans fat as in Hungary, and 28 times as much as in Denmark, where trans fats are restricted. At KFC, the pattern was reversed, with Hungary's product containing twice the trans fat of the New York product. Even within the U.S. there was variation, with fries in New York containing 30% more trans fat than those from Atlanta.[63]
| Food type | Trans fat content |
|---|---|
| butter | 2 to 7 g |
| whole milk | 0.07 to 0.1 g |
| animal fat | 0 to 5 g[48] |
| ground beef | 1 g |



High levels of TFAs have been recorded in popular "fast food" meals.[41] An analysis of samples of McDonald's French fries collected in 2004 and 2005 found that fries served in New York City contained twice as much trans fat as in Hungary, and 28 times as much as in Denmark, where trans fats are restricted. For Kentucky Fried Chicken products, the pattern was reversed: the Hungarian product containing twice the trans fat of the New York product. Even within the United States, there was variation, with fries in New York containing 30% more trans fat than those from Atlanta.[64]
Breast milk
[edit]It has been established that trans fats in human breast milk fluctuate with maternal consumption of trans fat, and that the amount of trans fats in the bloodstream of breastfed infants fluctuates with the amounts found in their milk. In 1999, reported percentages of trans fats (compared to total fats) in human milk ranged from 1% in Spain, 2% in France, 4% in Germany, and 7% in Canada and the U.S.[65]
Regulatory action
[edit]In the last few decades, there has been substantial amount of regulation in many countries, limiting trans fat contents of industrialized and commercial food products.
In light of recognized evidence and scientific agreement, nutritional authorities consider all trans fats equally harmful for health and recommend that their consumption be reduced to trace amounts.[1][2][4][5][7][10] In 2003, the WHO recommended that trans fats make up no more than 0.9% of a person's diet[48] and, in 2018, introduced a 6-step guide to eliminate industrially-produced trans-fatty acids from the global food supply.[66]
The National Academy of Sciences (NAS) advises the U.S. and Canadian governments on nutritional science for use in public policy and product labeling programs. Their 2002 Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids[67] contains their findings and recommendations regarding consumption of trans fat.[68]
Their recommendations are based on two key facts. First, "trans fatty acids are not essential and provide no known benefit to human health",[69] whether of animal or plant origin.[70] Second, given their documented effects on the LDL/HDL ratio,[71] the NAS concluded "that dietary trans fatty acids are more deleterious with respect to coronary artery disease than saturated fatty acids". A 2006 review stated "from a nutritional standpoint, the consumption of trans fatty acids results in considerable potential harm but no apparent benefit."[44]
Because of these facts and concerns, the NAS has concluded there is no safe level of trans fat consumption. There is no adequate level, recommended daily amount or tolerable upper limit for trans fats. This is because any incremental increase in trans fat intake increases the risk of coronary artery disease.[71]
Despite this concern, the NAS dietary recommendations have not included eliminating trans fat from the diet. This is because trans fat is naturally present in many animal foods in trace quantities, and thus its removal from ordinary diets might introduce undesirable side effects and nutritional imbalances. The NAS has, thus, "recommended that trans fatty acid consumption be as low as possible while consuming a nutritionally adequate diet".[72] Like the NAS, the WHO has tried to balance public health goals with a practical level of trans fat consumption, recommending in 2003 that trans fats be limited to less than 1% of overall energy intake.[48]
Health effects
[edit]While trans fatty acids (popularly called "trans fats") are edible, they have been implicated in many health problems.[73]
Cardiovascular disease
[edit]The primary health risk identified for trans fat consumption is an elevated risk of coronary artery disease (CAD).[44][74][75][76] A 1994 study estimated that over 30,000 cardiac deaths per year in the United States are attributable to the consumption of trans fats.[20] By 2006 upper estimates of 100,000 deaths were suggested.[77]
Major evidence for the effect of trans fat on CAD comes from the Nurses' Health Study – a cohort study that has been following 120,000 female nurses since its inception in 1976. In this study, Hu and colleagues analyzed data from 900 coronary events from the study's population during 14 years of followup. He determined that a nurse's CAD risk roughly doubled (relative risk of 1.93, CI: 1.43 to 2.61) for each 2% increase in trans fat calories consumed (instead of carbohydrate calories). By contrast, for each 5% increase in saturated fat calories (instead of carbohydrate calories) there was a 17% increase in risk (relative risk of 1.17, CI: 0.97 to 1.41). "The replacement of saturated fat or trans unsaturated fat by cis (unhydrogenated) unsaturated fats was associated with larger reductions in risk than an isocaloric replacement by carbohydrates."[78] Hu also reports on the benefits of reducing trans fat consumption. Replacing 2% of food energy from trans fat with non-trans unsaturated fats more than halves the risk of CAD (53%). By comparison, replacing a larger 5% of food energy from saturated fat with non-trans unsaturated fats reduces the risk of CAD by 43%.[78]
Another study considered deaths due to CAD, with consumption of trans fats being linked to an increase in mortality, and consumption of polyunsaturated fats being linked to a decrease in mortality.[76][79]
Analytical data (blood work)
[edit]Consuming trans fats has been shown to increase the risk of coronary artery disease in part by raising levels of low-density lipoprotein (LDL, often termed "bad cholesterol"), lowering levels of high-density lipoprotein (HDL, often termed "good cholesterol"), increasing triglycerides in the bloodstream and promoting systemic inflammation.[69][71]
Trans fat has been found to act like saturated in raising the blood level of LDL ("bad cholesterol"); but, unlike saturated fat, it also decreases levels of HDL ("good cholesterol"). The net increase in LDL/HDL ratio with trans fat, a widely accepted indicator of risk for coronary artery disease, is approximately double that due to saturated fat.[80][81][82] One randomized crossover study published in 2003 comparing the effect of eating a meal on blood lipids of (relatively) cis and trans-fat-rich meals showed that cholesteryl ester transfer (CET) was 28% higher after the trans meal than after the cis meal and that lipoprotein concentrations were enriched in apolipoprotein(a) after the trans meals.[83]
The citokyne test is a potentially more reliable indicator of CAD risk, although is still being studied.[76] A study of over 700 nurses showed that those in the highest quartile of trans fat consumption had blood levels of C-reactive protein (CRP) that were 73% higher than those in the lowest quartile.[84]
Intake of dietary trans fat perturbs the body's ability to metabolize essential fatty acids (EFAs, including omega-3) leading to changes in the phospholipid fatty acid composition of the arterial walls, thereby raising risk of coronary artery disease.[85]
There are two accepted tests that measure an individual's risk for coronary artery disease, both blood tests. The first considers ratios of two types of cholesterol, the other the amount of a cell-signalling cytokine called C-reactive protein. The effect of trans fat consumption has been documented on each as follows:
- Cholesterol ratio: This ratio compares the levels of LDL to HDL. Trans fat behaves like saturated fat by raising the level of LDL, but, unlike saturated fat, it has the additional effect of decreasing levels of HDL. The net increase in LDL/HDL ratio with trans fat is approximately double that due to saturated fat.[86][87][88] (Higher ratios are worse.) One randomized crossover study published in 2003 comparing the effect of eating a meal on blood lipids of (relatively) cis and trans fat rich meals showed that cholesteryl ester transfer (CET) was 28% higher after the trans meal than after the cis meal and that lipoprotein concentrations were enriched in apolipoprotein(a) after the trans meals.[89]
- C-reactive protein (CRP): A study of over 700 nurses showed that those in the highest quartile of trans fat consumption had blood levels of CRP that were 73% higher than those in the lowest quartile.[90]
Biochemical mechanisms
[edit]The mechanisms through which trans fatty acids contribute to coronary artery disease are fairly well understood. The mechanism for their effects on diabetes is still under investigation. They may impair the metabolism of long-chain polyunsaturated fatty acids (LCPUFAs).[91] However, maternal pregnancy trans fatty acid intake has been inversely associated with LCPUFAs levels in infants at birth thought to underlie the positive association between breastfeeding and intelligence.[92]
Trans fats are processed by the liver differently than other fats. They may cause liver dysfunction by interfering with delta 6 desaturase, an enzyme involved in converting essential fatty acids to arachidonic acid and prostaglandins, both of which are important to the functioning of cells.[93]
Intake of dietary trans fat disrupts the body's ability to metabolize essential fatty acids (EFAs, including Omega-3) leading to changes in the phospholipid fatty acid composition of the arterial walls, thereby raising risk of coronary artery disease.[94]
The major evidence for the effect of trans fat on CAD comes from the Nurses' Health Study – a cohort study that has been following 120,000 female nurses since its inception in 1976. In this study, Hu and colleagues analyzed data from 900 coronary events from the study's population during 14 years of followup. He determined that a nurse's CAD risk roughly doubled (relative risk of 1.93, CI: 1.43 to 2.61) for each 2% increase in trans fat calories consumed (instead of carbohydrate calories). By contrast, for each 5% increase in saturated fat calories (instead of carbohydrate calories) there was a 17% increase in risk (relative risk of 1.17, CI: 0.97 to 1.41). "The replacement of saturated fat or trans unsaturated fat by cis (unhydrogenated) unsaturated fats was associated with larger reductions in risk than an isocaloric replacement by carbohydrates."[78] Hu also reports on the benefits of reducing trans fat consumption. Replacing 2% of food energy from trans fat with non-trans unsaturated fats more than halves the risk of CAD (53%). By comparison, replacing a larger 5% of food energy from saturated fat with non-trans unsaturated fats reduces the risk of CAD by 43%.[78]
Another study considered deaths due to CAD, with consumption of trans fats being linked to an increase in mortality, and consumption of polyunsaturated fats being linked to a decrease in mortality.[95]
Other health risks
[edit]Scientific studies have examined other negative effects of industrial trans fat beyond cardiovascular disease, with the next most studied area being type-2 diabetes.
- Alzheimer's disease: A study published in Archives of Neurology in February 2003 suggested that the intake of both trans fats and saturated fats promote the development of Alzheimer disease,[96] although not confirmed in an animal model.[97] It has been found that trans fats impaired memory and learning in middle-age rats. The trans-fat eating rats' brains had fewer proteins critical to healthy neurological function and inflammation in and around the hippocampus, the part of the brain responsible for learning and memory. These are the exact types of changes normally seen at the onset of Alzheimer's, but seen after six weeks, even though the rats were still young.[98] A systematic review of five articles based on four prospective cohort studies of individuals did not find a robust association between their intake of trans fatty acids and development of Alzheimer's disease (or several other forms of dementia). The review based this conclusion on finding that 4 of the 5 reports appeared biased and therefore recommended more well-designed prospective studies to clarify this issue.[99]
- Cancer: In 2007 the American Cancer Society stated that a relationship between trans fats and cancer "has not been determined."[100] One study has found a positive connection between trans fat and prostate cancer.[101] However, a larger study found a correlation between trans fats and a significant decrease in high-grade prostate cancer.[102] An increased intake of trans fatty acids may raise the risk of breast cancer by 75%, suggest the results from the French part of the European Prospective Investigation into Cancer and Nutrition.[103][104]
- Diabetes: There is a growing concern that the risk of type 2 diabetes increases with trans fat consumption.[9] However, consensus has not been reached.[44] For example, one study found that risk is higher for those in the highest quartile of trans fat consumption.[105] Another study has found no diabetes risk once other factors such as total fat intake and BMI were accounted for.[106]
- Obesity: Research indicates that trans fat may increase weight gain and abdominal fat, despite a similar caloric intake.[107] A 6-year experiment revealed that monkeys fed a trans fat diet gained 7.2% of their body weight, as compared to 1.8% for monkeys on a mono-unsaturated fat diet.[108][109] Although obesity is frequently linked to trans fat in the popular media,[110] this is generally in the context of eating too many calories; there is not a strong scientific consensus connecting trans fat and obesity, although the 6-year experiment did find such a link, concluding that "under controlled feeding conditions, long-term TFA consumption was an independent factor in weight gain. TFAs enhanced intra-abdominal deposition of fat, even in the absence of caloric excess, and were associated with insulin resistance, with evidence that there is impaired post-insulin receptor binding signal transduction."[109]
- Liver dysfunction: Trans fats are metabolized differently by the liver than other fats and interfere with delta 6 desaturase. Delta 6 desaturase is an enzyme involved in converting essential fatty acids to arachidonic acid and prostaglandins, both of which are important to the functioning of cells.[111]
- Infertility in women: One 2007 study found, "Each 2% increase in the intake of energy from trans unsaturated fats, as opposed to that from carbohydrates, was associated with a 73% greater risk of ovulatory infertility...".[112]
- Major depressive disorder: Spanish researchers analysed the diets of 12,059 people over six years and found that those who ate the most trans fats had a 48 per cent higher risk of depression than those who did not eat trans fats.[113] One mechanism may be trans-fats' substitution for docosahexaenoic acid (DHA) levels in the orbitofrontal cortex (OFC). Very high intake of trans-fatty acids (43% of total fat) in mice from 2 to 16 months of age was associated with lowered DHA levels in the brain (p=0.001).[97] When the brains of 15 major depressive subjects who had committed suicide were examined post-mortem and compared against 27 age-matched controls, the suicidal brains were found to have 16% less (male average) to 32% less (female average) DHA in the OFC. The OFC controls reward, reward expectation, and empathy (all of which are reduced in depressive mood disorders) and regulates the limbic system.[114]
- Behavioral irritability and aggression: a 2012 observational analysis of subjects of an earlier study found a strong relation between dietary trans fat acids and self-reported behavioral aggression and irritability, suggesting but not establishing causality.[115]
- Diminished memory: In a 2015 article, researchers re-analyzing results from the 1999-2005 UCSD Statin Study argue that "greater dietary trans fatty acid consumption is linked to worse word memory in adults during years of high productivity."[116]
- Acne: According to a 2015 study, trans fats are one of several components of Western pattern diets which promote acne, along with carbohydrates with high glycemic load such as refined sugars or refined starches, milk and dairy products, and saturated fats, while omega-3 fatty acids, which reduce acne, are deficient in Western pattern diets.[117]
Food industry response
[edit]Manufacturer response
[edit]Palm oil, a natural oil extracted from the fruit of oil palm trees that is semi-solid at room temperature (15–25 degrees Celsius), can potentially serve as a substitute for partially hydrogenated fats in baking and processed food applications, although there is disagreement about whether replacing partially hydrogenated fats with palm oil confers any health benefits. A 2006 study supported by the National Institutes of Health and the USDA Agricultural Research Service concluded that palm oil is not a safe substitute for partially hydrogenated fats (trans fats) in the food industry, because palm oil results in adverse changes in the blood concentrations of LDL and apolipoprotein B just as trans fat does.[118][119]
In May 2003, BanTransFats.com Inc., a U.S. non-profit corporation, filed a lawsuit against the food manufacturer Kraft Foods in an attempt to force Kraft to remove trans fats from the Oreo cookie. The lawsuit was withdrawn when Kraft agreed to work on ways to find a substitute for the trans fat in the Oreo.
The J.M. Smucker Company, then the American manufacturer of Crisco (the original partially hydrogenated vegetable shortening), in 2004 released a new formulation made from solid saturated palm oil cut with soybean oil and sunflower oil. This blend yielded an equivalent shortening much like the prior partially hydrogenated Crisco, and was labelled zero grams of trans fat per 1 tablespoon serving (as compared with 1.5 grams per tablespoon of original Crisco).[120] As of 24 January 2007, Smucker said that all Crisco shortening products in the US had been reformulated to contain less than one gram of trans fat per serving while keeping saturated fat content less than butter.[121] The separately marketed trans fat free version introduced in 2004 was discontinued.
On 22 May 2004, Unilever, the corporate descendant of Joseph Crosfield & Sons (the original producer of Wilhelm Normann's hydrogenation hardened oils) announced that they had eliminated trans fats from all their margarine products in Canada, including their flagship Becel brand.[122]
Agribusiness giant Bunge Limited, through their Bunge Oils division, produce an NT product line of non-hydrogenated oils, margarines and shortenings, made from corn, canola, and soy oils.[123]
Major users' response
[edit]Beginning around 2000, as the scientific evidence and public concern about trans fat increased, major American users of trans fat began to switch to safer alternatives. The process received a large boost in 2003 when the FDA announced it would require trans fat labeling on packaged food starting in 2006. Packaged food companies then faced the choice of either eliminating trans fat from their products, or having to declare the trans fat on their nutrition label. Lawsuits in the U.S. against trans fat users also encouraged its removal.
Major American fast food chains including McDonald's, Burger King, KFC and Wendy's reduced and then removed partially hydrogenated oils (containing artificial trans fats) by 2009. This was a major step toward trans fat removal, as french fries were one of the largest sources of trans fat in the American diet, with a large serving of fries typically having about 6 grams of trans fat until around 2007.[124][125][126][127][128][129][130][131]
Two other events were important in the removal of trans fat. First, in 2013 the FDA announced it planned to completely ban artificial trans fat in the form of partially hydrogenated oil. Second, soon after this, Walmart informed its suppliers they needed to remove trans fat by 2015 if they wanted to continue to sell their products at its stores. As Walmart is the largest brick-and-mortar retailer in the U.S., mainstream food brands had little choice but to comply.[132]
These reformulations can be partly attributed to 2006 Center for Science in the Public Interest class action complaints, and to New York's restaurant trans fat ban, with companies such as McDonald's stating they would not be selling a unique product just for New York customers but would implement a nationwide or worldwide change.[133][134][135]
See also
[edit]Notes
[edit]References
[edit]- ^ a b c d "Trans fats in foods: A new regulation for EU consumers" (PDF). European Commission. 2019. Retrieved 24 January 2025.
- ^ a b c d "Trans fat". US Food and Drug Administration. 30 April 2024. Retrieved 24 January 2025.
- ^ Gormley JJ, Juturu V (2010). "Partially Hydrogenated Fats in the US Diet and Their Role in Disease". In De Meester F, Zibadi S, Watson RR (eds.). Modern Dietary Fat Intakes in Disease Promotion. Nutrition and Health. Totowa, NJ: Humana Press. pp. 85–94. doi:10.1007/978-1-60327-571-2_5. ISBN 978-1-60327-571-2.
- ^ a b "Scientific opinion on dietary reference values for fats". EFSA Journal. 8 (3). EFSA Panel on Dietetic Products, Nutrition, and Allergies: 1461. 2010. doi:10.2903/j.efsa.2010.1461.
- ^ a b UK National Health Service (14 April 2023). "Fat: the facts". Retrieved 24 January 2025.
- ^ a b c Brouwer IA, Wanders AJ, Katan MB (March 2010). "Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans - a quantitative review". PLOS ONE. 5 (3): e9434. Bibcode:2010PLoSO...5.9434B. doi:10.1371/journal.pone.0009434. PMC 2830458. PMID 20209147.
- ^ a b c "Canadian Ban on Trans Fats Comes into Force Today". Health Canada, Government of Canada. 17 September 2018. Retrieved 24 January 2025.
- ^ a b Sergey Volkov (1 February 2024). "REPLACE Trans fat-free". World Health Organization. Retrieved 11 April 2024.
- ^ a b de Souza RJ, Mente A, Maroleanu A, et al. (11 August 2015). "Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies". BMJ (Clinical Research Ed.). 351: h3978. doi:10.1136/bmj.h3978. ISSN 1756-1833. PMC 4532752. PMID 26268692.
- ^ a b "Trans fatty acids". Food Standards Australia-New Zealand. May 2017. Retrieved 24 January 2025.
- ^ a b Ghebreyesus TA, Frieden T (2021). "Eliminating trans fat from foods will save lives without changing the taste: Only our hearts will know the difference". World Health Organization. Retrieved 22 July 2023.
- ^ "Butter, salted". FoodData Central. 1 April 2019. Retrieved 11 October 2024.
- ^ a b Alves SP, Vahmani P, Mapiye C, et al. (2021). "Trans-10 18:1 in ruminant meats: A review". Lipids. 32 (6): 539–562. doi:10.1002/lipd.12324. PMID 34608647. S2CID 238356805.
- ^ Kuhnt K, Baehr M, Rohrer C, et al. (October 2011). "Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods". European Journal of Lipid Science and Technology. 113 (10): 1281–1292. doi:10.1002/ejlt.201100037. PMC 3229980. PMID 22164125.
- ^ Teresa Tarrago-Trani M, Phillips KM, Lemar LE, et al. (2006). "New and Existing Oils and Fats Used in Products with Reduced Trans-Fatty Acid Content" (PDF). Journal of the American Dietetic Association. 106 (6): 867–880. doi:10.1016/j.jada.2006.03.010. PMID 16720128.
- ^ a b Chatgilialoglu C, Ferreri C, Melchiorre M, et al. (2014). "Lipid Geometrical Isomerism: From Chemistry to Biology and Diagnostics". Chemical Reviews. 114 (1): 255–284. doi:10.1021/cr4002287. PMID 24050531.
- ^ a b "About Trans Fat and Partially Hydrogenated Oils" (PDF). Center for Science in the Public Interest.
- ^ a b c d "Tentative Determination Regarding Partially Hydrogenated Oils". Federal Register. 8 November 2013. 2013-26854, Vol. 78, No. 217. Archived from the original on 6 April 2014. Retrieved 8 November 2013.
- ^ Ascherio A, Stampfer MJ, Willett WC (1999). "Trans fatty acids and coronary heart disease". The New England Journal of Medicine. 340 (25): 1994–1998. doi:10.1056/NEJM199906243402511. PMID 10379026. Archived from the original on 3 September 2006. Retrieved 14 September 2006.
- ^ a b Willett WC, Ascherio A (May 1994). "Trans fatty acids: are the effects only marginal?". American Journal of Public Health. 84 (5): 722–4. doi:10.2105/AJPH.84.5.722. PMC 1615057. PMID 8179036.
- ^ Schleifer D (January 2012). "The perfect solution: How trans fats became the healthy replacement for saturated fats". Technology and Culture. 53 (1): 94–119. doi:10.1353/tech.2012.0018. JSTOR 41475458. PMID 22530389. S2CID 26343964.
- ^ References:
- Centre for Science in the Public Interest (1999). "Health Advocates Tell Top Restaurants 'Get an Oil Change'". Retrieved 22 July 2023.
- Jacobson MF (2004). "Good riddance to Trans!". Nutrition Action Healthletter. No. 31. Center for Science in the Public Interest. Cited in.[21]
- Centre for Science in the Public Interest (2018). "Artificial Trans Fat: A Timeline". Retrieved 22 July 2023.
- ^ "Lawsuit dropped as Oreo looks to drop the fat". CNN. 14 May 2003. Retrieved 14 July 2011.
- ^ "CSPI withdraws from lawsuit after KFC cuts trans fat". Centre for Science in the Public Interest. 2006. Retrieved 22 July 2023.
- ^ L'Abbé MR, Stender S, Skeaff CM, et al. (2009). "Approaches to removing trans fats from the food supply in industrialized and developing countries". European Journal of Clinical Nutrition. 63 (Suppl 1): S50–7. doi:10.1038/ejcn.2009.14. PMC 2830089. PMID 19190645.
- ^ "Information Sheet: REPLACE trans fat: an action package to eliminate industrially produced trans-fatty acids". World Health Organization. 2021. Retrieved 22 July 2023.
- ^ Regulation: 21 CFR 101.9 (c)(2)(ii). Food and Drug Administration (11 July 2003). "21 CFR Part 101. Food labeling; trans fatty acids in nutrition labeling; consumer research to consider nutrient content and health claims and possible footnote or disclosure statements; final rule and proposed rule" (PDF). National Archives and Records Administration. Archived from the original (PDF) on 3 January 2007. Retrieved 18 January 2007.
- ^ "FDA acts to provide better information to consumers on trans fats". Food and Drug Administration. Archived from the original on 25 June 2005. Retrieved 26 July 2005.
- ^ "Newswise: most consumers misinterpret meaning of trans fat information on Nutrition Facts panel". Retrieved 19 June 2008.
- ^ Shockman L (5 December 2005). "Trans fat: 'Zero' foods add up". Toledo Blade. Archived from the original on 21 June 2009. Retrieved 18 January 2007.
- ^ Casimir C. Akoh, David B. Min., eds. (2002). Food lipids: chemistry, nutrition, and biotechnology. New York: M. Dekker. pp. 1–2. ISBN 978-0-8247-0749-1.
- ^ ""fatty acid"". IUPAC Gold book. International Union of Pure and Applied Chemistry. 2014. doi:10.1351/goldbook.F02330.
- ^ Alonso L, Fontecha J, Lozada L, et al. (May 1999). "Fatty acid composition of caprine milk: major, branched-chain, and trans fatty acids". Journal of Dairy Science. 82 (5): 878–84. doi:10.3168/jds.S0022-0302(99)75306-3. hdl:10261/113439. PMID 10342226.
- ^ Thomas A (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_173. ISBN 978-3-527-30673-2.
- ^ Martin CA, Milinsk MC, Visentainer JV, et al. (June 2007). "Trans fatty acid-forming processes in foods: a review". Anais da Academia Brasileira de Ciências. 79 (2): 343–50. doi:10.1590/S0001-37652007000200015. PMID 17625687.
- ^ Udo Erasmus; Fats that heal, Fats that Kill, Alive books, 1993 edition, Pages 13-19.
- ^ a b "Section 7: Biochemistry". Handbook of chemistry and physics. 2007–2008 (88th ed.). Taylor and Francis. 2007. Archived from the original (PDF) on 24 July 2017. Retrieved 19 November 2007.
- ^ Gormley JJ, Juturu V (2010). "Partially Hydrogenated Fats in the US Diet and Their Role in Disease". In De Meester F, Zibadi S, Watson RR (eds.). Modern Dietary Fat Intakes in Disease Promotion. Nutrition and Health. Totowa, NJ: Humana Press. pp. 85–94. doi:10.1007/978-1-60327-571-2_5. ISBN 978-1-60327-571-2.
- ^ Hill JW, Kolb DK (2007). Chemistry for changing times. Pearson / Prentice Hall. ISBN 978-0-13-605449-8.
{{cite book}}: CS1 maint: overridden setting (link) - ^ Ashok C, Ajit V (2009). "Chapter 4: Fatty acids". A Textbook of Molecular Biotechnology. I. K. International Pvt. p. 181. ISBN 978-93-80026-37-4.
{{cite book}}: CS1 maint: overridden setting (link) - ^ a b c d Valenzuela A, Morgado N (1999). "Trans fatty acid isomers in human health and in the food industry". Biological Research. 32 (4): 273–87. doi:10.4067/s0716-97601999000400007. PMID 10983247.
- ^ Eller FJ, List GR, Teel JA, et al. (July 2005). "Preparation of spread oils meeting U.S. Food and Drug Administration Labeling requirements for trans fatty acids via pressure-controlled hydrogenation". Journal of Agricultural and Food Chemistry. 53 (15): 5982–4. doi:10.1021/jf047849+. PMID 16028984.
- ^ Tarrago-Trani MT, Phillips KM, Lemar LE, et al. (June 2006). "New and existing oils and fats used in products with reduced trans-fatty acid content". Journal of the American Dietetic Association. 106 (6): 867–80. doi:10.1016/j.jada.2006.03.010. PMID 16720128.
- ^ a b c d e f g Mozaffarian D, Katan MB, Ascherio A, et al. (2006). "Trans Fatty Acids and Cardiovascular Disease". New England Journal of Medicine. 354 (15): 1601–1613. doi:10.1056/NEJMra054035. PMID 16611951.
- ^ a b c d Trans Fat Task Force (June 2006). TRANSforming the Food Supply. Trans Fat Task Force. ISBN 0-662-43689-X. Retrieved 7 January 2007.
- ^ Kuhnt K, Baehr M, Rohrer C, et al. (October 2011). "Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods". European Journal of Lipid Science and Technology. 113 (10): 1281–1292. doi:10.1002/ejlt.201100037. PMC 3229980. PMID 22164125.
- ^ Kummerow FA, Kummerow JM (2008). Cholesterol Won't Kill You, But Trans Fat Could. Trafford. ISBN 978-1-4251-3808-0.
- ^ a b c d e Trans Fat Task Force (June 2006). TRANSforming the Food Supply. Trans Fat Task Force. ISBN 0-662-43689-X. Retrieved 7 January 2007.
- ^ "DIETA DETOX ✅ QUÉ ES Y SUS 13 PODEROSOS BENEFICIOS". 24 October 2019.
- ^ National Dairy Council (18 June 2004). "comments on 'Docket No. 2003N-0076 Food Labeling: Trans Fatty Acids in Nutrition Labeling'" (PDF). Retrieved 7 January 2007.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 424.[permanent dead link]
- ^ a b Tricon S, Burdge GC, Kew S, et al. (September 2004). "Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans". The American Journal of Clinical Nutrition. 80 (3): 614–20. doi:10.1093/ajcn/80.3.614. PMID 15321800.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Zulet MA, Marti A, Parra MD, et al. (September 2005). "Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health". Journal of Physiology and Biochemistry. 61 (3): 483–94. doi:10.1007/BF03168454. PMID 16440602. S2CID 32082565.
- ^ "National Nutrient Database for Standard Reference Release 28". United States Department of Agriculture.[dead link]
- ^ National Dairy Council (18 June 2004). "comments on 'Docket No. 2003N-0076 Food Labeling: Trans Fatty Acids in Nutrition Labeling'" (PDF). Food and Drug Administration. Archived from the original (PDF) on 16 May 2005. Retrieved 7 January 2007.
- ^ Zulet MA, Marti A, Parra MD, et al. (September 2005). "Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health". Journal of Physiology and Biochemistry. 61 (3): 483–94. doi:10.1007/BF03168454. PMID 16440602. S2CID 32082565.
- ^ Ashok C, Ajit V (2009). "Chapter 4: Fatty acids". A Textbook of Molecular Biotechnology. I. K. International Pvt. p. 181. ISBN 978-93-80026-37-4.
- ^ a b Tarrago-Trani MT, Phillips KM, Lemar LE, et al. (June 2006). "New and existing oils and fats used in products with reduced trans-fatty acid content". Journal of the American Dietetic Association. 106 (6): 867–80. doi:10.1016/j.jada.2006.03.010. PMID 16720128.
- ^ "Heart Foundation: Butter has 20 times the trans fats of marg | Australian Food News". www.ausfoodnews.com.au.
- ^ Riserus U (2006). "Trans fatty acids, insulin sensitivity and type 2 diabetes". Scandinavian Journal of Food and Nutrition. 50 (4): 161–165. doi:10.1080/17482970601133114.
- ^ Hunter JE (2005). "Dietary levels of trans fatty acids" basis for health concerns and industry efforts to limit use". Nutrition Research. 25 (5): 499–513. doi:10.1016/j.nutres.2005.04.002.
- ^ NYC Board of Health. "Board of Health Approves Regulation to Phase Out Artificial Trans Fat: FAQ". Archived from the original on 6 October 2006. Retrieved 7 January 2007.
- ^ "What's in that french fry? Fat varies by city". NBC News. 12 April 2006. Retrieved 7 January 2007.[dead link] AP story concerning Stender S, Dyerberg J, Astrup A (April 2006). "High levels of industrially produced trans fat in popular fast foods". N. Engl. J. Med. 354 (15): 1650–2. doi:10.1056/NEJMc052959. PMID 16611965.
- ^ "What's in that french fry? Fat varies by city". NBC News. 12 April 2006. Archived from the original on 17 October 2015. Retrieved 7 January 2007. AP story concerning Stender S, Dyerberg J, Astrup A (April 2006). "High levels of industrially produced trans fat in popular fast foods". N. Engl. J. Med. 354 (15): 1650–2. doi:10.1056/NEJMc052959. PMID 16611965.
- ^ Innis SM, King DJ (September 1999). "trans Fatty acids in human milk are inversely associated with concentrations of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants". The American Journal of Clinical Nutrition. 70 (3): 383–90. doi:10.1093/ajcn/70.3.383. PMID 10479201.
- ^ "WHO plan to eliminate industrially-produced trans-fatty acids from global food supply" (Press release). World Health Organization. 14 May 2018.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. i. Archived from the original on 18 September 2006.
- ^ Summary Archived 2007-06-25 at the Wayback Machine.
- ^ a b Food and nutrition board, institute of medicine of the national academies (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academies Press. pp. 423. doi:10.17226/10490. ISBN 978-0-309-08525-0.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 447.[permanent dead link]
- ^ a b c Food and nutrition board, institute of medicine of the national academies (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academies Press. p. 504.[permanent dead link]
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 424.[permanent dead link]
- ^ Menaa F, Menaa A, Menaa B, et al. (June 2013). "Trans-fatty acids, dangerous bonds for health? A background review paper of their use, consumption, health implications and regulation in France". European Journal of Nutrition. 52 (4): 1289–302. doi:10.1007/s00394-012-0484-4. PMID 23269652. S2CID 206968361.
- ^ "Fats and Cholesterol" Archived 2016-11-18 at the Wayback Machine, Harvard School of Public Health. Retrieved 02-11-16.
- ^ Government of the United Kingdom (1996): "Schedule 7: Nutrition labelling Archived 2013-03-17 at the Wayback Machine". In Food Labelling Regulations 1996 Archived 2013-09-21 at the Wayback Machine. Accessed on 2020-08-09.
- ^ a b c Trans Fat Task Force (June 2006). "TRANSforming the Food Supply, Appendix 9iii". Archived from the original on 25 February 2007. Retrieved 9 January 2007. (Consultation on the health implications of alternatives to trans fatty acids: Summary of Responses from Experts)
- ^ Zaloga GP, Harvey KA, Stillwell W, et al. (October 2006). "Trans fatty acids and coronary heart disease". Nutrition in Clinical Practice. 21 (5): 505–12. doi:10.1177/0115426506021005505. PMID 16998148.
- ^ a b c d Hu FB, Stampfer MJ, Manson JE, et al. (November 1997). "Dietary fat intake and the risk of coronary heart disease in women". The New England Journal of Medicine. 337 (21): 1491–9. doi:10.1056/NEJM199711203372102. PMID 9366580.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Oh K, Hu FB, Manson JE, et al. (April 2005). "Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses' health study". American Journal of Epidemiology. 161 (7): 672–9. doi:10.1093/aje/kwi085. PMID 15781956.
- ^ Ascherio A, Katan MB, Zock PL, et al. (June 1999). "Trans fatty acids and coronary heart disease". The New England Journal of Medicine. 340 (25): 1994–8. doi:10.1056/NEJM199906243402511. PMID 10379026. S2CID 30165590.
- ^ Mensink RP, Katan MB (August 1990). "Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects". The New England Journal of Medicine. 323 (7): 439–45. doi:10.1056/NEJM199008163230703. PMID 2374566.
- ^ Mensink RP, Zock PL, Kester AD, et al. (May 2003). "Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials". The American Journal of Clinical Nutrition. 77 (5): 1146–55. doi:10.1093/ajcn/77.5.1146. PMID 12716665.
- ^ Gatto LM, Sullivan DR, Samman S (May 2003). "Postprandial effects of dietary trans fatty acids on apolipoprotein(a) and cholesteryl ester transfer". The American Journal of Clinical Nutrition. 77 (5): 1119–24. doi:10.1093/ajcn/77.5.1119. PMID 12716661.
- ^ Lopez-Garcia E, Schulze MB, Meigs JB, et al. (March 2005). "Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction". The Journal of Nutrition. 135 (3): 562–6. doi:10.1093/jn/135.3.562. PMID 15735094.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Kummerow FA, Zhou Q, Mahfouz MM, et al. (April 2004). "Trans fatty acids in hydrogenated fat inhibited the synthesis of the polyunsaturated fatty acids in the phospholipid of arterial cells". Life Sciences. 74 (22): 2707–23. doi:10.1016/j.lfs.2003.10.013. PMID 15043986.
- ^ Ascherio A, Katan MB, Zock PL, et al. (June 1999). "Trans fatty acids and coronary heart disease". The New England Journal of Medicine. 340 (25): 1994–8. doi:10.1056/NEJM199906243402511. PMID 10379026. S2CID 30165590.
- ^ Mensink RP, Katan MB (August 1990). "Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects". The New England Journal of Medicine. 323 (7): 439–45. doi:10.1056/NEJM199008163230703. PMID 2374566.
- ^ Mensink RP, Zock PL, Kester AD, et al. (May 2003). "Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials". The American Journal of Clinical Nutrition. 77 (5): 1146–55. doi:10.1093/ajcn/77.5.1146. PMID 12716665.
- ^ Gatto LM, Sullivan DR, Samman S (May 2003). "Postprandial effects of dietary trans fatty acids on apolipoprotein(a) and cholesteryl ester transfer". The American Journal of Clinical Nutrition. 77 (5): 1119–24. doi:10.1093/ajcn/77.5.1119. PMID 12716661.
- ^ Lopez-Garcia E, Schulze MB, Meigs JB, et al. (March 2005). "Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction". The Journal of Nutrition. 135 (3): 562–6. doi:10.1093/jn/135.3.562. PMID 15735094.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Mojska H (2003). "Influence of trans fatty acids on infant and fetus development". Acta Microbiologica Polonica. 52 Suppl: 67–74. PMID 15058815.
- ^ Koletzko B, Decsi T (October 1997). "Metabolic aspects of trans fatty acids". Clinical Nutrition. 16 (5): 229–37. doi:10.1016/s0261-5614(97)80034-9. PMID 16844601.
- ^ Mahfouz M (1981). "Effect of dietary trans fatty acids on the delta 5, delta 6 and delta 9 desaturases of rat liver microsomes in vivo". Acta Biologica et Medica Germanica. 40 (12): 1699–1705. PMID 7345825.
- ^ Kummerow FA, Zhou Q, Mahfouz MM, et al. (April 2004). "Trans fatty acids in hydrogenated fat inhibited the synthesis of the polyunsaturated fatty acids in the phospholipid of arterial cells". Life Sciences. 74 (22): 2707–23. doi:10.1016/j.lfs.2003.10.013. PMID 15043986.
- ^ Oh K, Hu FB, Manson JE, et al. (April 2005). "Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses' health study". American Journal of Epidemiology. 161 (7): 672–9. doi:10.1093/aje/kwi085. PMID 15781956.
- ^ Morris MC, Evans DA, Bienias JL, et al. (February 2003). "Dietary fats and the risk of incident Alzheimer disease". Archives of Neurology. 60 (2): 194–200. doi:10.1001/archneur.60.2.194. PMID 12580703.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ a b Phivilay A, Julien C, Tremblay C, et al. (March 2009). "High dietary consumption of trans fatty acids decreases brain docosahexaenoic acid but does not alter amyloid-beta and tau pathologies in the 3xTg-AD model of Alzheimer's disease". Neuroscience. 159 (1): 296–307. doi:10.1016/j.neuroscience.2008.12.006. PMID 19135506. S2CID 35748183.
- ^ Granholm AC, Bimonte-Nelson HA, Moore AB, et al. (June 2008). "Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat". Journal of Alzheimer's Disease. 14 (2): 133–45. doi:10.3233/JAD-2008-14202. PMC 2670571. PMID 18560126.
- ^ Nwaru BI, Dierkes J, Ramel A, et al. (2022). "Quality of dietary fat and risk of Alzheimer's disease and dementia in adults aged ≥50 years: a systematic review". Food & Nutrition Research. 66. doi:10.29219/fnr.v66.8629. PMC 9338447. PMID 35950105.
- ^ American Cancer Society. "Common questions about diet and cancer". Archived from the original on 13 April 2010. Retrieved 9 January 2007.
- ^ Chavarro J, Stampfer M, Campos H, et al. (1 April 2006). "A prospective study of blood trans fatty acid levels and risk of prostate cancer". Proc. Amer. Assoc. Cancer Res. 47 (1): 943. Retrieved 9 January 2007.
- ^ Brasky TM, Till C, White E, et al. (June 2011). "Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial". American Journal of Epidemiology. 173 (12): 1429–39. doi:10.1093/aje/kwr027. PMC 3145396. PMID 21518693.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ "Breast cancer: a role for trans fatty acids?". World Health Organization (Press release). 11 April 2008. Archived from the original on 13 April 2008.
- ^ Chajès V, Thiébaut AC, Rotival M, et al. (June 2008). "Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC Study". American Journal of Epidemiology. 167 (11): 1312–20. doi:10.1093/aje/kwn069. PMC 2679982. PMID 18390841.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Hu FB, van Dam RM, Liu S (July 2001). "Diet and risk of Type II diabetes: the role of types of fat and carbohydrate". Diabetologia. 44 (7): 805–17. doi:10.1007/s001250100547. PMID 11508264.
- ^ van Dam RM, Willett WC, Rimm EB, et al. (March 2002). "Dietary fat and meat intake in relation to risk of type 2 diabetes in men". Diabetes Care. 25 (3): 417–24. doi:10.2337/diacare.25.3.417. PMID 11874924.
- ^ Gosline A (12 June 2006). "Why fast foods are bad, even in moderation". New Scientist. Retrieved 9 January 2007.
- ^ "Six years of fast-food fats supersizes monkeys". New Scientist (2556): 21. 17 June 2006.
- ^ a b Kavanagh K, Jones KL, Sawyer J, et al. (July 2007). "Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys". Obesity. 15 (7): 1675–84. doi:10.1038/oby.2007.200. PMID 17636085. S2CID 4835948.
- ^ Thompson TG. "Trans Fat Press Conference". Archived from the original on 9 July 2006., US Secretary of health and human services
- ^ Mahfouz M (1981). "Effect of dietary trans fatty acids on the delta 5, delta 6 and delta 9 desaturases of rat liver microsomes in vivo". Acta Biologica et Medica Germanica. 40 (12): 1699–1705. PMID 7345825.
- ^ Chavarro JE, Rich-Edwards JW, Rosner BA, et al. (January 2007). "Dietary fatty acid intakes and the risk of ovulatory infertility". The American Journal of Clinical Nutrition. 85 (1): 231–7. doi:10.1093/ajcn/85.1.231. PMID 17209201.
- ^ Roan S (28 January 2011). "Trans fats and saturated fats could contribute to depression". The Sydney Morning Herald. Retrieved 8 February 2011.
- ^ McNamara RK, Hahn CG, Jandacek R, et al. (July 2007). "Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder". Biological Psychiatry. 62 (1): 17–24. doi:10.1016/j.biopsych.2006.08.026. PMID 17188654. S2CID 32898004.
- ^ Golomb BA, Evans MA, White HL, et al. (2012). "Trans fat consumption and aggression". PLOS ONE. 7 (3): e32175. Bibcode:2012PLoSO...732175G. doi:10.1371/journal.pone.0032175. PMC 3293881. PMID 22403632.
- ^ Golomb BA, Bui AK (2015). "A Fat to Forget: Trans Fat Consumption and Memory". PLOS ONE. 10 (6): e0128129. Bibcode:2015PLoSO..1028129G. doi:10.1371/journal.pone.0128129. PMC 4470692. PMID 26083739.
- ^ Melnik BC (15 July 2015). Weinberg J (ed.). "Linking diet to acne metabolomics, inflammation, and comedogenesis: an update". Clinical, Cosmetic and Investigational Dermatology. 8: 371–88. doi:10.2147/CCID.S69135. PMC 4507494. PMID 26203267.
- ^ Vega-López S, Ausman LM, Jalbert SM, et al. (July 2006). "Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects". The American Journal of Clinical Nutrition. 84 (1): 54–62. doi:10.1093/ajcn/84.1.54. PMID 16825681.
- ^ "Palm oil not a healthy substitute for trans fats, study finds". ScienceDaily. 11 May 2009. Retrieved 12 May 2010.
- ^ "Crisco 0 grams trans fat per serving all-vegetable shortening". Archived from the original on 15 October 2006. Retrieved 18 January 2007.
- ^ ""Crisco Frequently Asked Questions". Archived from the original on 27 September 2007.
- ^ "List of Canadian industry actions to reduce transfats". Food & Consumer Products of Canada (FCPC). Archived from the original on 27 September 2007. Retrieved 13 September 2007.
- ^ "Welcome to TransFatSolutions.com powered by Bunge". Transfatsolutions.com. Retrieved 14 July 2011.
- ^ Urban LE (2014). "Temporal Trends in Fast-Food Restaurant Energy, Sodium, Saturated Fat, and Trans Fat Content, United States, 1996–2013". Preventing Chronic Disease. 11: E229. doi:10.5888/pcd11.140202. ISSN 1545-1151. PMC 4283359. PMID 25551184.
- ^ "Trans Fat Food Industry Response". News-Medical.net. 24 March 2010.
- ^ "KFC Sued for Fouling Chicken with Partially Hydrogenated Oil: Lawsuit Aimed at Eliminating, or Disclosing Use of Artery-Clogging Frying Oil" (Press release). Center for Science in the Public Interest. 12 June 2006. Retrieved 18 January 2007.
- ^ "Class action complaint" (PDF). 12 June 2006. Retrieved 18 January 2007.
- ^ Burros M (14 June 2006). "KFC Is Sued Over the Use of Trans Fats in Its Cooking". The New York Times. Retrieved 18 January 2007.
- ^ "KFC announces switch to zero trans fat cooking oil following two-year test for same great taste" (Press release). KFC. 30 October 2006. Archived from the original on 19 January 2007. Retrieved 18 January 2007.
- ^ "KFC Canada phasing in zero grams trans fat menu in all 786 restaurants nationally early in the new year" (Press release). KFC Canada. 30 October 2006. Archived from the original on 18 February 2007. Retrieved 18 January 2007.
- ^ "Wendy's Significantly Cuts Trans Fats – Switch to New Cooking Oil Under Way" (Press release). Wendy's. 8 June 2006. Archived from the original on 9 November 2006. Retrieved 11 April 2013.
- ^ "McDonald's finally picks trans-fat-free oil". NBC News. 30 January 2007.[dead link]
- ^ "Chick-fil-A Removes Trans Fats from Menu" (Press release). NBC News. 10 October 2008.
- ^ Sainz A (6 July 2006). "Burger King to Use Trans-Fat-Free Oil" (Press release). Associated Press. Archived from the original on 29 September 2007. Retrieved 6 July 2007.
- ^ Nutrition Information Archived 21 July 2015 at the Wayback Machine. Ihop.com (2015). Retrieved 22 August 2015.
Further reading
[edit]- Dijkstra A, Hamilton RJ, Wolf H, eds. (2008). Trans Fatty Acids. Blackwell. ISBN 978-1-4051-5691-2.
- Jang ES, Jung MY, Min DB (2005). "Hydrogenation for Low Trans and High Conjugated Fatty Acids" (PDF). Comprehensive Reviews in Food Science and Food Safety. 1. Archived from the original (PDF) on 17 December 2008.
External links
[edit]- "Ban the Trans: These Sorry Lipids Should Go Away"
- Center for Science in the Public Interest Trans Fat Page
- Harvard School of Public Health webpage on trans-fat
- "Labeling & Nutrition – Guidance for Industry: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, Health Claims; Small Entity Compliance Guide". Center for Food Safety and Applied Nutrition. August 2003. Archived from the original on 26 October 2013. Retrieved 6 April 2014.
- Federal Register – 68 FR 41433 11 July 2003: Food Labeling: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, and Health Claims